Liquid Sodium Acetate Trihydrate Liquid Sodium Acetate Trihydrate Art
Where to Buy Sodium Acetate
Wondering where you can purchase Sodium Acetate? Permit united states help, we acquit Sodium Acetate Anhydrous that is a Technical course. You will desire to choose a supplier that has been effectually a while and can provide product speedily. Soapgoods has been in business since 2006 and unremarkably ships items out the same or next business organization day. Nosotros besides take a 45-day satisfaction guarantee.
Where is Sodium Acetate from?
Sodium acetate is a manufactured product, manufactured industrially for consumer use. It may exist unnaturally produced, even so, it is fabricated from the sodium salt of acerb acid which is an organic chemical compound.

It is possible to make small batches of Sodium Acetate at dwelling house, you lot tin find some ideas on making your own Sodium Acetate here
Geographically our Sodium Acetate is Made in the U.s.
How fast tin I get it
We Guarantee Your order ships out the same or next business day! This ways in the South East you volition have your order in 1 to 3 business concern days, in the North E normally 3 to 4 days and in the West unremarkably 4 to 5 days. For total details on shipping and processing times please see our expected delivery times.
These times are based on business days, not including weekends or holidays.
FedEx Delivery Map
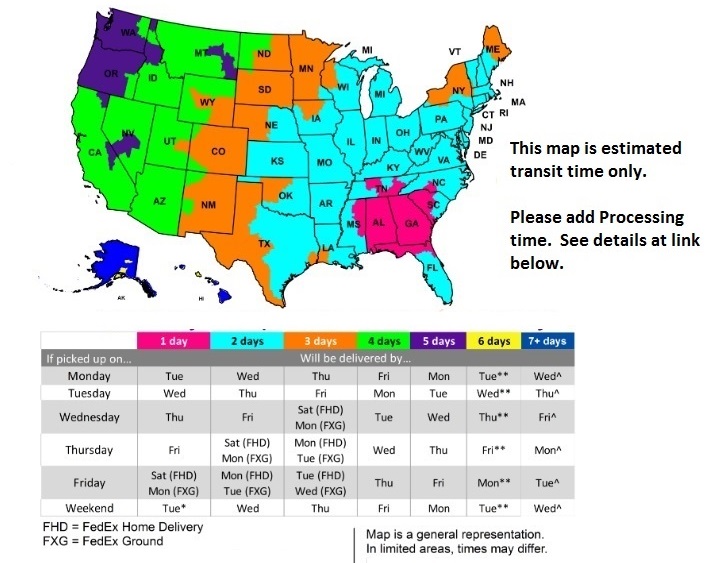
For Processing Times Click Hither
USPS Delivery Map
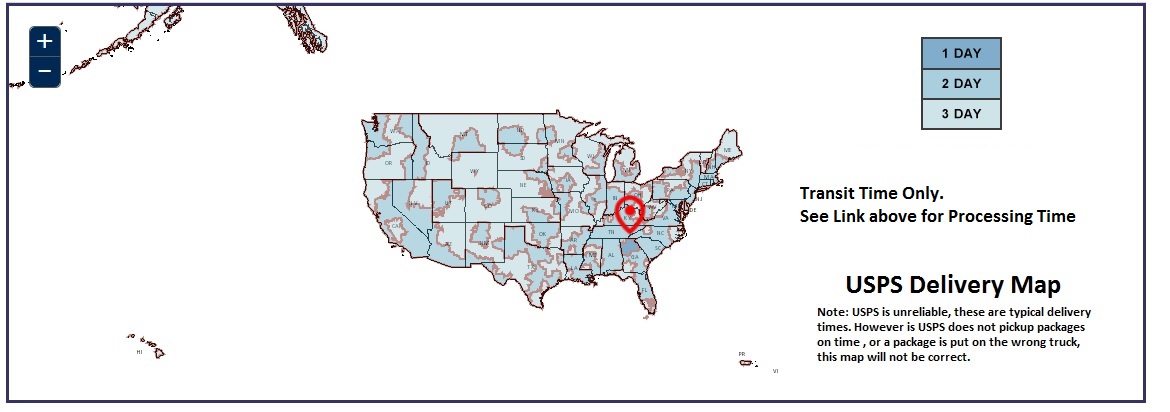
How Does it Ship
Our Sodium Acetate is packed in sturdy, recloseable plastic bags. The larger sizes, 50 lbs and higher are packaged in the industrial-grade newspaper bags lined in plastic, typical of bulk chemical packaging.
Which Grade practise y'all need
Sodium Acetate is bachelor in several types and grades including Anhydrous and Trihydrate too equally pharmaceutical and technical grades. The Anhydrous is normally a pulverization while the Trihydrate is typically a granular crystal. Too, the Trihydrate is very soluble in water compared to the anhydrous version. The anhydrous offers different characteristics including low dustiness, higher reactivity, college density, and improved free-menstruation-ability. The molecular weight of the Trihydrate is 136.08 g/mol compared to the anhydrous at 82.04 thou/mol. The Cas No for Sodium Acetate Anhydrous is 127-09-03, the Trihydrate is identified by CAS No. 6131-ninety-iv
Is Sodium Acetate Unsafe
In full general Sodium Acetate is a adequately safe chemical, in fact, it is on the FDA'southward list of Generally Recognized equally Safe (Gras) chemicals. In relatively small levels it is benign in the environment, and not considered harmful.
In fact, it is a major component of Sodium diacetate that is a very mutual preservative and flavor enhancer used in many foods. It is however considered to be an eye and a picayune bit of a skin irritant in some situations.
The other caution is that Sodium Acetate can be combustible if brought into contact with open flames, it also reacts if mixed with oxidizers such as nitrc acid or potassium nitrate.
How Long can you lot Keep Sodium Acetate
Sodium Acetate, if stored in ideal storage weather condition can last anywhere from 2 to 4 years or more. Information technology is all-time to go on it dry, cool and out of the sun, an closed container would work peachy. Regular testing subsequently the 2 years will ensure the sodium acetate still works well in your application. However if at all possible it is best to only purchase quantities that will be used in 2 years to ensure platonic performance.
How is Sodium Acetate Made
Sodium acetate is manufactured by reacting acetic acid (vinegar) with sodium hydroxide. There are other manufacturing methods including using sodium carbonate instead of the sodium hydroxide. A manufacturer could even create sodium acetate by combining vinegar and sodium bicarbonate (baking Soda)
You lot can actually make sodium Acetate at home using Baking Soda, Vinegar, Coffee Filters and a spoon. Your results may vary and we propose caution but you lot tin can find more information on making sodium acetate here
Sodium Acetate Uses
Sodium acetate is commonly used to assist increase the life of concrete through helping to seal it confronting h2o permeation. Information technology has been used every bit a substitute for the epoxy to this ends.
Also commonly used with Acetic Acrid to deed every bit a buffer to maintain a consequent pH level between 4 -6.
It is also commonly used in the tanning, to increase the penetration of the dyes and tanning products. It also helps to increase the rate at which the tan is absorbed.
In the TEXTILE manufacture, sodium acetate is considered a dye and color intermediate, with specific utilize as a mordant in the dyeing process, it is also used in the in material industries to neutralize sulfuric acid waste material streams. Because of its ability to remove insoluble calcium salts, sodium acetate is further used by the textile industry to amend the wearing quality of finished fabrics.
Information technology is also unremarkably used in the petrochemical industries.
If you want to make your own hot ice y'all will demand Sodium Acetate as it is a major ingredient. You lot tin can notice a formula to brand hot ice below.
It is besides commonly used as a polymerization catalyst and in detergents, for its thermal energy storage characteristics, You can detect Sodium Acetate used in conjunction with Sodium Diacetate to increment flavors of various foods.
Is Sodium Acetate Natural?
Sodium Acetate is a common salt of Acetic Acrid and sodium hydroxide. Acetic acid is an organic compound, then it really depends on if the individual would consider it natural in that circumstance. Acetic acid is products naturally equally fruits brainstorm to spoil.
Quick Details:
- Synonyms: Sodium Acetate Anhydrous
- Grade: Technical Grade
- CAS: 127-09-3
- Appearance: White Powder
- Solubility: Soluble
- Natural or Synthetic: Synthetically produced from natural production.
- Recommended Retest or Shelf life: 2 Years
- Storage: Store in a cool dry out, night place. Airtight is recommended
What blazon of Sodium Acetate is this
We bear Sodium Acetate Anhydrous, a technical form.
How exercise I know this is Good
Our Sodium Acetate is manufactured to the highest standards past one of the largest manufacturers in the world. Headquartered right hither in the USA. They have been in functioning for almost 100 years. We also take a 45 day, return period for our products including our Sodium Acetate.
How to Use Sodium Acetate for Dry out Ice
What yous need to do is saturate the water with sodium acetate at a high temperature. There are 2 ways of getting this.
Heat water to about 60 deg C, then add sodium acetate until it will no longer dissolve. Pour the liquid into a vessel, making sure you DO NOT transfer any solid into the vessel. Stick the solution into the fridge until it is cooled to fridge temperature or even room temperature. At this point, the solution is supercooled, and any trigger tin can induce crystallization.
Make a saturated solution of sodium acetate at room temperature (i.eastward. add acetate until y'all are left with an undissolved layer). Oestrus the solution to 60 deg C, which causes the residual to deliquesce. Once the solution is completely clear, pour it into a vessel and stick it in the fridge, equally in method ane.
Method one will requite you lot more than crystals when touched, but any solid transferred will ruin the experiment. Method ii volition always piece of work but generates fewer crystals.
There are no other specifics. You need to make a supersaturated solution, and can not transfer whatever solid material from the saturate.
How to Make Hot Ice With Sodium Acetate
Things You'll Need:
- Sodium acetate
- Small sauce pan
- Water
Make full a saucepan volition h2o and heat it until information technology is near-boiling. Boiling is likewise hot, just a pocket-sized amount of simmering is okay. The chief affair is to make the water hot.
Dissolve as much sodium acetate into the water as possible. The more you deliquesce into the water, the denser the crystals will be when they form the hot ice. Keep stirring in as much equally possible until you tin can no longer go the sodium acetate to dissolve in the h2o. It is important to stir the mixture constantly at this stage.
Know that when the mixture is completely dissolved, pour it into a glass or other container. Make sure to just pour the h2o with dissolved sodium acetate into the drinking glass. If any sodium acetate remains undissolved, leave information technology in the lesser of the pan every bit you pour.
Identify the glass in your fridge to allow the water to absurd.
Be enlightened that after the mixture has cooled, pour some into a tray, mold or whatever container you lot wish to concur the hot ice. As you pour it into the tray, note that it remains in liquid form.
Just touching the solution will not instigate the reaction, you must introduce a "seed" crystal. To practise this, lightly moisten your fingertip and then a couple of dry/un-dissolved crystals will stick at that place, now y'all're ready to touch the solution. Touch the top of the liquid with the tip of your finger. You exercise non need to dip your finger or concord it on the liquid. Merely a quick tap is all that is needed. Hot ice crystals will instantly class where you lot touch and volition spread through the mixture until every drop of liquid is in a frozen merely hot crystalline country. In simply one 2d yous volition have hot ice.
Source: https://www.soapgoods.com/Sodium-Acetate-Anhydrous-p-770.html
0 Response to "Liquid Sodium Acetate Trihydrate Liquid Sodium Acetate Trihydrate Art"
ارسال یک نظر